Why Biohaven Stock Plummeted by More Than 15% Today
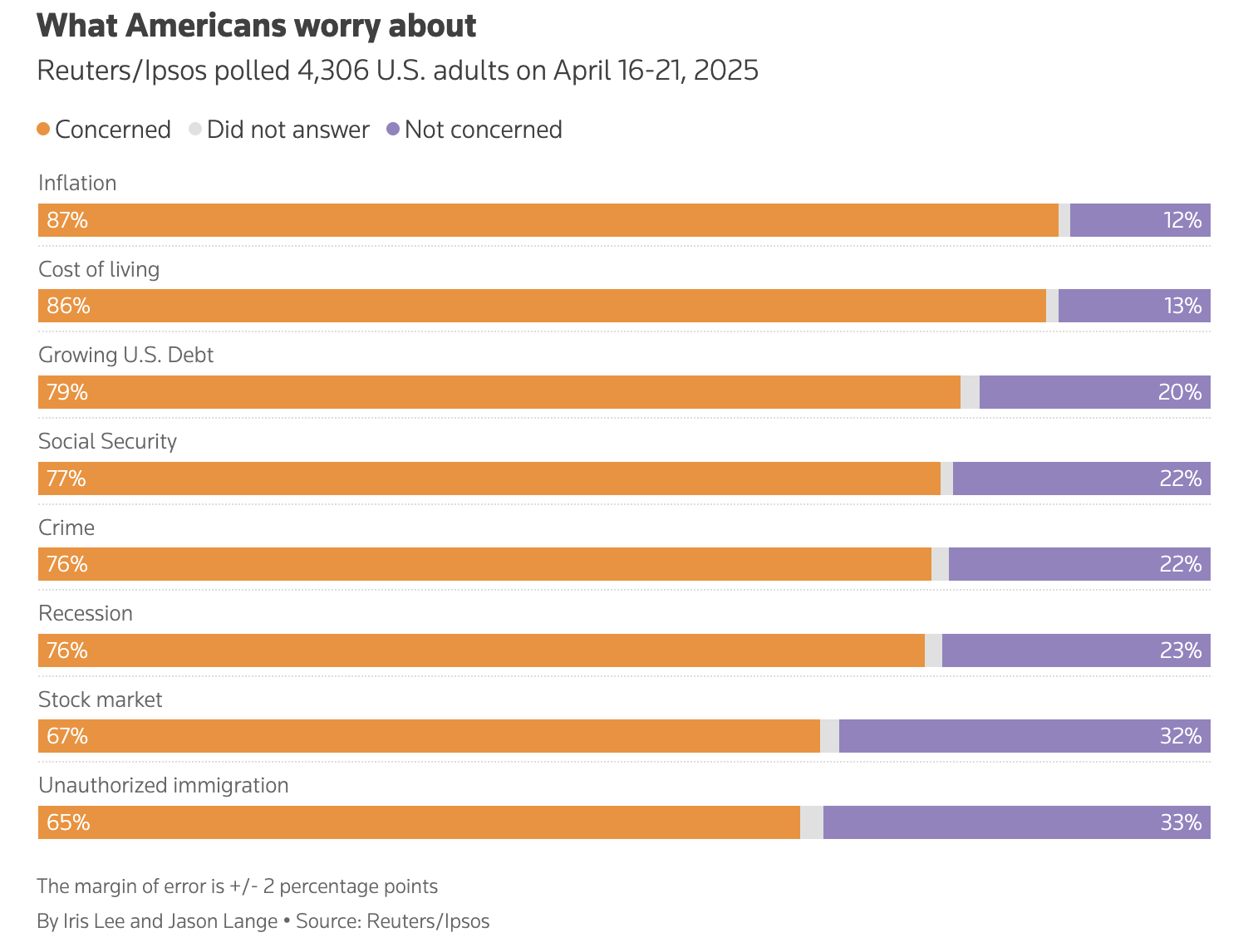
On Friday's news that Biohaven (NYSE: BHVN) withdrew its application for a top investigational drug from an important regulator, investors aggressively traded out of the stock. The clinical-stage biotech was one of the biggest decliners in its industry, exiting Friday with a share price decline of over 15%. This was during a session that was essentially bullish; the S&P 500 index closed 0.6% higher. That news came not from Biohaven itself, but from the European Medicines Agency (EMA, the pharmaceutical regulator for the 27-country European Union). In a statement, the EMA revealed that a subsidiary of the company withdrew its application for marketing authorization for troriluzole, branded as Dazluma. It made that move on March 24, the agency added. Biohaven originally applied for such authorization for Dazluma toward the end of 2023. The investigational drug targets a rare genetic affliction of nerve cells called spinocerebellar ataxia type 3. Continue reading

On Friday's news that Biohaven (NYSE: BHVN) withdrew its application for a top investigational drug from an important regulator, investors aggressively traded out of the stock. The clinical-stage biotech was one of the biggest decliners in its industry, exiting Friday with a share price decline of over 15%. This was during a session that was essentially bullish; the S&P 500 index closed 0.6% higher.
That news came not from Biohaven itself, but from the European Medicines Agency (EMA, the pharmaceutical regulator for the 27-country European Union). In a statement, the EMA revealed that a subsidiary of the company withdrew its application for marketing authorization for troriluzole, branded as Dazluma. It made that move on March 24, the agency added.
Biohaven originally applied for such authorization for Dazluma toward the end of 2023. The investigational drug targets a rare genetic affliction of nerve cells called spinocerebellar ataxia type 3.