Weight-loss pill breakthrough sends Eli Lilly stock surging
The major pharmaceutical company has a new obesity drug on the horizon.

It's little secret that America has a weight problem. For decades, Americans have embraced and abandoned countless weight-loss strategies, including fad diets, that have proven ineffective long term.
Despite our best efforts, more people are at risk of health complications resulting from obesity, including type 2 diabetes and heart disease.
Related: UnitedHealth stock tumbles after Medicare Advantage changes hit outlook
Shockingly, about three-quarters of the U.S. population is considered obese or overweight, according to the National Institutes for Health. Over 35 million have type-2 diabetes, and 96 million Americans have prediabetes, according to the Centers for Disease Control and Prevention (CDC).
The costs associated with America's weight problem are staggering, totaling $173 billion annually.
Fortunately, emerging weight-loss medicines have won FDA approval, providing millions of Americans with their best shot yet at weight loss.
Still, weight-loss drugs are pricey, and many insurance companies won't pay for them. Also, medicines specifically approved for weight loss use are injected, rather than taken as a pill, making them less desirable to many.
That may soon change, given recent progress announced by Eli Lilly.
Wegovy and Mounjaro battle for weight-loss dominance
Novo Nordisk’s (NVO) Wegovy, which is also sold in a lower dose as the diabetes drug Ozempic, was the first FDA-approved weight-loss drug to hit the market in 2021. The active ingredient in those drugs (semaglutide) is also approved as a pill for diabetes under the brand name Rybelsus.
Eli Lilly (LLY) joined the weight-loss battle in 2023 when it secured approval for Zepbound, which is also sold as Mounjaro for diabetics.
Related: Struggling drugstore chain may file Chapter 11 bankruptcy again
Novo and Eli Lilly's drugs target glucagon-like peptide-1. More easily said, GLP-1, a hormone that slows digestion, makes people feel fuller for longer. Zepbound also targets the GIP hormone, which also slows digestion.
Doctors use the body mass index (BMI), which measures body fat in adults by dividing weight by height, to determine whether someone is a healthy weight. Generally, a BMI between 18.5 and 25 is desirable, a BMI between 25 to 30 is considered overweight, and a BMI above 30 is considered obese.
Zepbound and Wegovy are approved for use in those with a BMI above 30 or those with a BMI above 27 with at least one other weight-related disease, such as hypertension.
The weight-loss results for the drugs are impressive.
Those who fall in the overweight and obese camps experience a higher rate of adverse health outcomes, including diabetes and cardiovascular disease.
In clinical trials, Wegovy reduced weight by 15% after 68 weeks. About one in three patients lost over 20% of their weight. Mounjaro patients saw average weight loss of 21% of body weight.
Related: Surprising decision sends weight-loss drug stock reeling
Eli Lilly reported in December that a head-to-head trial comparing Zepbound to Wegovy showed, on average, Zepbound patients lost 20.2% of their weight, compared to 13.7% for patients taking Wegovy.
The substantial weight loss seen in clinical trials has turned these drugs into mega-blockbusters.
In 2024, Novo reported Wegovy revenue surged 86% year-over-year to about $8 billion. Eli Lilly's Zepbound revenue totaled $4.95 billion last year, as sales soared following its 2023 approval.
Eli Lilly's plans for a once-daily weight loss pill may pan out
On April 17, Eli Lilly reported results from a phase 3 clinical trial evaluating an oral dose of a new compound, orforglipron. Unlike Zepbound, it only targets GLP-1.
In the study, the once-daily pill reduced weight by an average of 16 pounds, or about 7.9% on average, making it the first oral GLP-1 to complete a phase 3 trial successfully.
Eli Lilly hopes to file for FDA approval of orforglipron in weight loss by the end of 2025, with a filing for approval for type 2 diabetes patients sometime in 2026.
That timeline should help it compete with Novo Nordisk, which said in February it planned to file its oral weight-loss drug for FDA approval in the first quarter.
If these drugs win the FDA okay, availability of once-daily weight loss pills in the battle against obesity will offer patients a potentially better option than off-and-on, yo-yo diets.
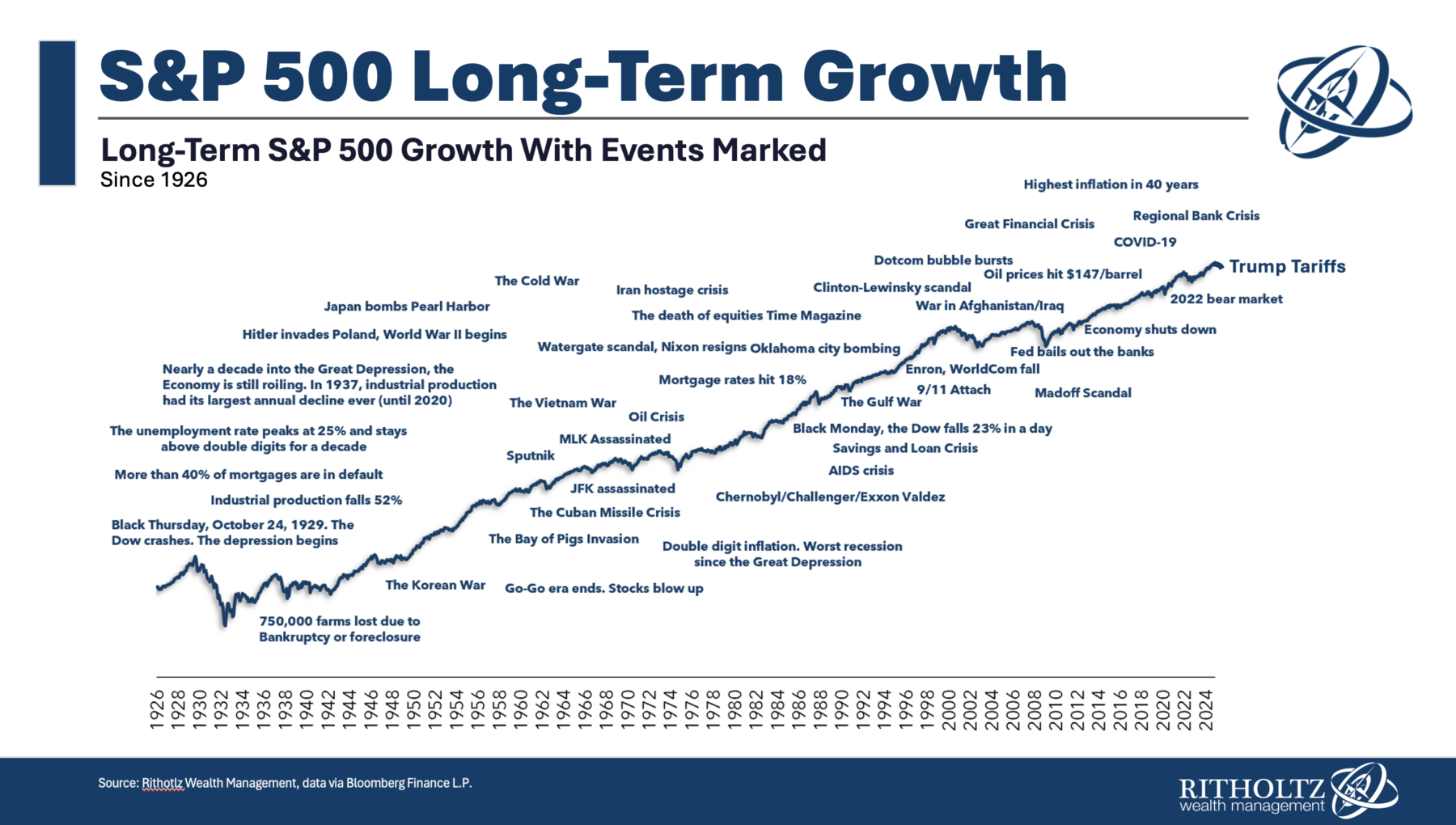
Unsurprisingly, Eli Lilly's stock price is soaring on the news, climbing 14% at midday on April 17. Novo Nordisk shares are down 7%.
Related: Veteran fund manager unveils eye-popping S&P 500 forecast