Why Shares in Amylyx Pharmaceuticals Soared This Week
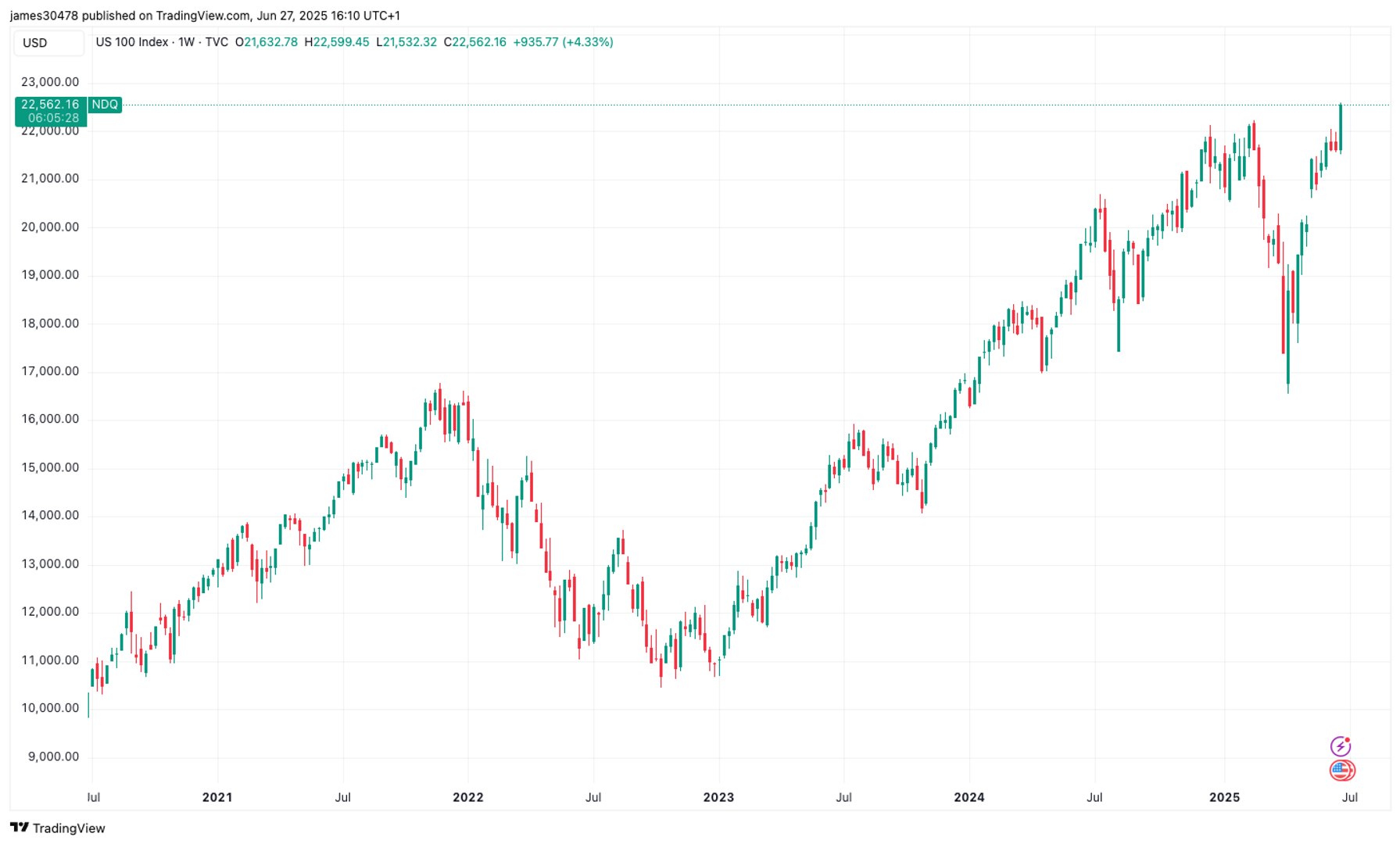
Shares in Amylyx Pharmaceuticals (NASDAQ: AMLX) were up almost 28% in the week to Friday morning. The move comes after an analyst at Guggenheim initiated coverage of the small-cap stock and issued a buy recommendation, accompanied by a price target of $17. For reference, the stock price at the time of writing is $6.50.The main excitement surrounds avexitide, a GLP-1 receptor antagonist, which Amylyx is currently testing in a phase 3 trial for post-bariatric hypoglycemia (PBH). According to Amylyx, about 8% of patients who have undergone bariatric (weight loss) surgery end up with PBH; this translates to 160,000 people living with PBH now. The Guggenheim analyst believes avexitide has blockbuster potential (usually implying sales of over $1 billion), and investors are hoping Amylyx can replicate the results of its phase 2 trial.Management anticipates that the phase 3 Lucidity trial will be completed in 2025, with headline results expected to be available in the first half of 2026. Continue reading

Shares in Amylyx Pharmaceuticals (NASDAQ: AMLX) were up almost 28% in the week to Friday morning. The move comes after an analyst at Guggenheim initiated coverage of the small-cap stock and issued a buy recommendation, accompanied by a price target of $17. For reference, the stock price at the time of writing is $6.50.
The main excitement surrounds avexitide, a GLP-1 receptor antagonist, which Amylyx is currently testing in a phase 3 trial for post-bariatric hypoglycemia (PBH). According to Amylyx, about 8% of patients who have undergone bariatric (weight loss) surgery end up with PBH; this translates to 160,000 people living with PBH now. The Guggenheim analyst believes avexitide has blockbuster potential (usually implying sales of over $1 billion), and investors are hoping Amylyx can replicate the results of its phase 2 trial.
Management anticipates that the phase 3 Lucidity trial will be completed in 2025, with headline results expected to be available in the first half of 2026.