Drug regulation – How does it work?
31st December 2022 Before laying into the drug regulators, and their inexorable move towards the dark side, I thought I should try to explain a bit more about who decides what drugs should be used, and for what conditions. Yes, I know, for most people this appears simple. The Federal Drugs Administration (FDA), in the […]
31st December 2022
Before laying into the drug regulators, and their inexorable move towards the dark side, I thought I should try to explain a bit more about who decides what drugs should be used, and for what conditions.
Yes, I know, for most people this appears simple. The Federal Drugs Administration (FDA), in the US, or the European Medicines Agency (EMA), for the European Union, approve drugs for use in human beings, and that’s pretty much that.
Other countries have their own drug evaluation agencies, but l have no intention of looking at them in any detail. Also, if the FDA and EMEA approve drugs, then they are pretty much waved through elsewhere. A statement that will no doubt be assailed by various parties but I stand by it.
In short, if these two big agencies say a drug is safe – and effective enough – it is given their stamp of approval. It is then allowed to be prescribed … pretty much worldwide. Therefore, yes, the FDA/EMA represent the first hurdle that needs to be cleared, or your drug is going nowhere.
However, this once formidable hurdle has been hammered down into an almost unnoticeable speed bump, sitting about one inch above the ground. To quote from Forbes magazine – as far back as 2015 ‘The FDA Is Basically Approving Everything. Here’s The Data To Prove It.’ 1
‘In 2008, companies asked for 134 approvals and got 75 of them, a 56% approval rate. That rate hovered steady in 2009 and 2010, and then rose to about 70% in 2011, 2012, and 2013. Last year it jumped to 77%, with 97 out of 126 requests for approval coming back positive. This year’s approval rate? It was 88%…
in reality, the FDA approval rate is more like 96%. Eliminating BioMedTracker’s counting of multiple uses for the same drug means FDA approved 23 drugs and rejected 1, Merck ’s anesthesia antidote, Bridion. Again, that means 19 of 20 new drug applications were approved.’
Drug companies have long since worked out how to neutralise the FDA and EMA. Which means that the big effort, nowadays, is made in working with other, increasingly important ‘agencies’, to achieve three main things:
- Market expansion
- New indications for use
- Co-opting ‘your’ drugs into the guidelines
I think of FDA approval as establishing the initial bridgehead in a seaborne invasion. Once you have landed, you can then spread out to take over the rest of the country. This has become a massive and resource intensive exercise and involves many other different ‘agencies’ that need to be brought to heel.
In the UK, for example, a clear second barrier to drug use is (or more accurately ‘was’) the National Institute for Clinical and Health Excellence (NICE). This ‘agency’ was set up to decide if a drug, or other medical intervention, provided good value for money.
If not, NICE said no, and the drug would not be approved. Doctors could still prescribe such drugs, but it was much frowned upon, and could result in various sanctions.
When it started, in 1999, NICE decreed that no healthcare intervention should cost more than thirty thousand pounds for each year of perfect quality life that it provided (approx. $40K). One year of perfect health is known as one quality adjusted life year (1 QALY). Calculating QALYs is fraught with assumptions and models, and suchlike, which I am not going into here.
Where did this thirty thousand figure come from? The truth is that was plucked from thin air. Strangely, this figure has never increased, in well over twenty years. It is inflation proof. It is also endlessly flexible. Think of it as the pot of gold at the end of the rainbow. You know it is there, but it can never truly be seen, or pinned down. This allows for endless fudging to take place, depending on how the media and politicians react to their decisions.
The first ever judgement of NICE was to turn down the anti-flu drug Relenza. I think it was just to show how ruthless and anti-industry they were going to be. A flag rammed into the ground. ‘We own this territory.’ This caused the CEO of Glaxo Smith Kline (GSK) to hurl his toys out of the pram. Various drug companies sent a letter to Tony Blair, the Prime Minster at the time:
“We warned that NICE’s activities could have worldwide repercussions for sales of the medicines concerned and that it could send out deeply damaging signals about the future rewards for innovation. We received repeated assurances from Ministers and from Sir Michael Rawlins (head of the NICE) that our concerns were well understood and that NICE would not operate as a fourth hurdle for new medicines. The landmark ruling on Relenza makes it crystal clear that our worst fears were fully justified,” it said.
The letter went on: “the emergence of NICE as a new obstacle to market entry serves to wipe out, at a stroke, a key element of the UK’s competitive advantage in the global pharmaceutical industry. It is self-evident that any savings to the NHS resulting from restricting access to new medicines will be insignificant when set alongside the potential loss of the UK’s current international standing in the pharmaceutical industry.”
“Much damage has already been done by NICE’s recommendation….the government must act swiftly now to limit and repair the damage by making clear that its response to NICE will take full account of the wider implications of its activities and that new medicines approved by the MCA will continue to have immediate access to the NHS,” said Dr McKillop.’ 2
I think GSK threatened to pull out of the UK altogether. In the medical world we call the ‘I am going to scweam and scweam and scweam until I am sick, sick, sick.’ business strategy.
However, it did not take long before the industry ceased their pitiful screaming and realised the NICE could be one of their most valuable assets. How so? Primarily because other countries do not really have a NICE equivalent, and many of them look to the judgements of NICE for guidance. If NICE say yes, then they will almost always say yes as well. Bingo.
Ergo, if you manage to get NICE to say that your drug it not only safe and effective, but also cost-effective. This opens the doors worldwide. The market is yours. And so, inevitably, NICE has gone the way of the FDA. They now approve, pretty much everything. Increasingly, they don’t even bother to let anyone know how they worked out their figures.
For example, we can take a look at the drug Inclisiran. This is a cholesterol lowering injectable drug, known as a PCSK9-Inhibitor. A number of different PCSK-9 inhibitors have reached the market recently. They all lower cholesterol (LDL) more than statins – hooray (or perhaps not). They are all, also, mind-bogglingly more expensive.
A year’s supply of a statin now costs about thirty pounds (forty dollars) per year. At least it does in the UK. On the other hand, a one-year supply of a PCSK-9 inhibitor costs around five thousand. Some cost a bit more, some less. However, they are all at least one hundred times more expensive than statins, more like two hundred.
I once idly calculated that if everyone taking a statin were to move over to a PCSK9- Inhibitor instead, it would cost the NHS around sixty billion pounds a year. Which would mean cancelling almost all other activities. Hip replacements … you must be joking, no money left for that nonsense. Cholesterol lowering is what the NHS now does. And nothing else!
At this point you might be asking yourself. How could a drug with very few additional ‘benefits’ to a statin possibly manage to get approved by NICE? How did anyone manage to work this one out? You may be glad to know that I am not going to go through all the complicated trial results, calculations and suchlike here.
But you can, if you wish, read it all for yourself in the ‘evidence’ section of the NICE report on Inclisiran. All two hundred and forty-three pages of it. And good luck with that.3
Over the years these NICE reports have become utterly bonkers. They are now so long, so jargon filled, with statistics filling the air. They are also so very, very, very, boring. Some may say that this is a strategy used to stop any objections to their decisions. Primarily, because no-one could possibly be bothered reading the damned thing. Bullshit baffles brains.
Little do they know that I occasionally rouse myself to look at NICE reviews in detail. Even though some parts are beyond me. Here is one very brief example of jargon-filled obfuscation taken from page sixty-five of the Inclisiran report:
‘The time-adjusted percentage change in LDL-C from baseline after Day 90 and up to Day 540 was calculated from the MMRM. Linear combinations of the estimated means after Day 90 and up to Day 540 were used to compare treatment effects.
Treatment effects from these 100 MMRM analyses were then combined using Rubin’s Method (100) via the SAS PROC MIANALYZE procedure. The difference in the least squares means between treatment groups and corresponding two-sided 95% CI was provided for hypothesis testing.’
- The MMRM analyses?
- Rubin’s method?
- The SAS PROC MIANALYZE procedure?
Search me guv.
To be honest I tend to skim these parts. This is on the basis that I have better things to do with my life than find out what the SAS PROC MIANALYZE procedure might be. Instead, I spend my time searching for the key facts that have been hidden away. The secret to the magic trick. The ‘Prestige.’
As with all magic tricks:
“The first part is called “The Pledge”. The magician shows you something ordinary: a deck of cards, a bird or a man. He shows you this object. Perhaps he asks you to inspect it to see if it is indeed real, unaltered, normal. But of course … it probably isn’t.”
“The second act is called “The Turn”. The magician takes the ordinary something and makes it do something extraordinary. Now you’re looking for the secret … but you won’t find it, because of course you’re not really looking. You don’t really want to know how it, say, disappeared. You want to be fooled.”
“But you wouldn’t clap yet. Because making something disappear isn’t enough; you have to bring it back. That’s why every magic trick has a third act, the hardest part, the part we call The Prestige.” 4
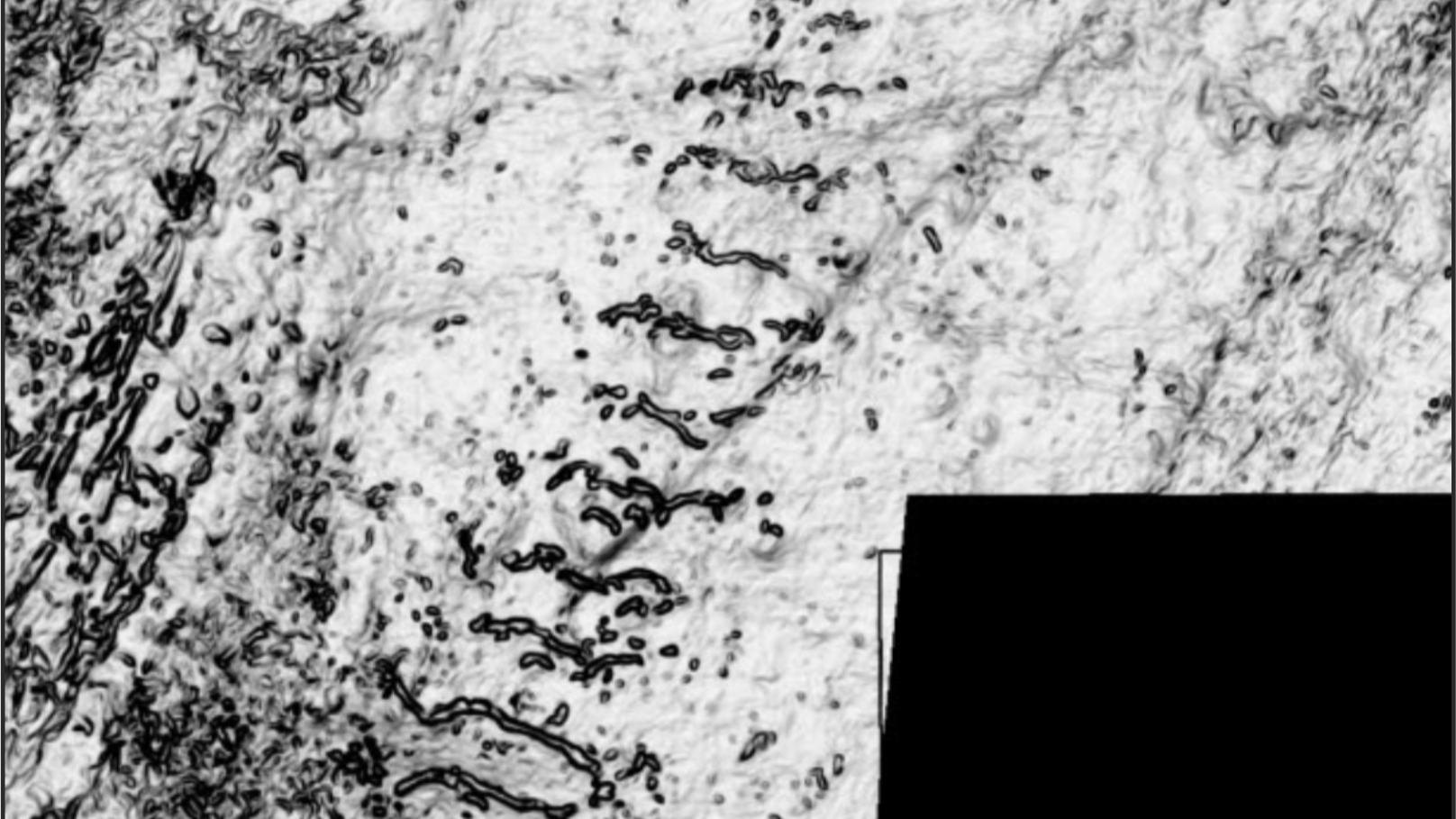
Where was the ‘Prestige’ with Inclisiran? I knew it was hidden somewhere deep within those two hundred and forty-three pages. My attention designed to be cunningly diverted by such things as the SAS PROC MIANALYZE procedure. Say what? My first clue as to where the Prestige lies can be found is on page one hundred and six (see below).

Just look at those thick black lines. Yes, here is a report by a tax-payer funded Government agency. But we are not allowed to see critical data. Such as, how many participants in the trial suffered an adverse event. Nor how many discontinued the drug and – perhaps most critically – how many died. Really, they are keeping all this a secret? Yes, indeed, they are. Here is another page I thought you might enjoy. It is page 112. It is a belter. All the information you need in one critical table

Oh no, it’s all been redacted – again. After this point there is page after page, after page, of black text and thick black lines. What does it actually say beneath the censored information?
We’re in the money
Come on, my honey
Let’s spend it, lend it,
Send it rolling around!
Moving on, the single most important thing for us to know, from a NICE report, is the following?
Is Inclisiran cost-effective? Or, to put it another way, can it provide more than 1QALY for each thirty thousand pounds it costs? In addition, can it really be that much more effective than statins. [In my opinion, nothing is more effective than statins. So, use nothing].
This, then, is the central NICE question. Is Inclisiran cost-effective, or not. I cannot answer this question, and nor can you – or anyone else outside NICE. Why not, you may ask. Well, to answer this question I present you with, but one small section, that looks at the cost-effectiveness of Inclisiran.
In this case cost-effectiveness on the treatment of Atherosclerotic Cardiovascular disease (ASCVD).

As you can see… you can’t see anything. You are not allowed to. This table can be found on page 211 by the way. Quite astonishingly, all the information on costs has been redacted. This table is followed by many other with all the figures redacted. How as this happened? Because Novartis will not allow NICE to show it to you.
What is the point of doing all this work, and publishing this enormous document, if all the critical information is to be kept secret? Kept secret from the very people who pay for all the damned work. NICE is taxpayer funded, its calculations should be transparent, and its decisions should be transparent. But they are not.
The simple fact is that the pharmaceutical industry has learned how to control NICE. It has become, like the FDA and the EMA, a ‘captured’ agency. On the outside it pretends to be a fully independent scourge of the pharmaceutical agency. In reality it does exactly what it is told – by the pharmaceutical industry.
In this case, the crowd goes wild, as the magician demonstrates his Inclisiran trick.
‘Ladies and gentlemen, here is a PCSK-9 inhibitor called Inclisiran. Look at it closely. Yes, examine it any way you like (note to self, make sure they don’t actually look at anything after page 100). It costs…. What does it cost Madam. Why cost is not the issue. What matters is whether or not it is cost-effective. Am I right, madam? My, your dress is so beautiful, and your hair. If I may say magnificent.’’
Woman nods and smiles.
‘Yes, you may be thinking … how can this drug possibly be cost-effective?’ Well, let me place this drug into the sealed box that we call NICE evaluation. Yes madam, a most impressive box indeed. Stamped with approval by, well, everyone. Yes, madam, everyone.’
Magician places Inclisiran into black box.
‘Now, we just need some money…. I shall stuff two million pounds into the box …’
Magician stuffs the box with money, then shakes it.
‘Hey presto.’ He opens the box. ‘Yes, as you can see Inclisiran is, indeed, cost-effective. Yes sir, it is indeed, magic … what’s that, you would like to see into the box yourself. Sorry, sir, we have to keep some of our secrets to ourself …. Yes, officer, if you could just take that gentleman out and arrest him for some reason or another…’
Nowadays, the tentacles of the pharmaceutical (and medical devices industry) wrap around far more agencies than just the FDA and EMA. And is not just NICE. It is the medical societies, the opinion leaders, the charities and – let us not forget – the politicians. All are caught up its deadly embrace. No-one escapes. If they do, they immediately become a conspiracy theorist.
In the next episode I shall turn my attention to the Universities, and those who work in them. Here lies, perhaps, the greatest source of power. A place where money can be converted into both academic and medical authority. Increasingly backed up by the force of law.
3: https://www.nice.org.uk/guidance/ta733/evidence/committee-papers-pdf-9258232573 4: https://observer.com/2020/09/the-prestige-christopher-nolan-magic-trick/









![[DEALS] iScanner App: Lifetime Subscription (79% off) & Other Deals Up To 98% Off – Offers End Soon!](https://www.javacodegeeks.com/wp-content/uploads/2012/12/jcg-logo.jpg)